The Xiang Group
Heterogeneous catalysis, Kinetics and Mechanism

Natural Gas Liquids (NGLs) Conversion
The current shale gas revolution in the United State has revitalized the research interest from both academia and industry in developing new and more efficient processes related to the conversion of natural gas and NGLs into high-value petrochemical products. As indicated in their 2016 workshop organized by the National Academies of Sciences, Engineering, and Medicine, the global energy structure, and chemicals industry might reshape from crude oil as their major source to natural gas due to the current shale gas revolution. Additionally, an NSF Center for Innovative and Strategic Transformation of Alkane Resources has been established recently.

Our research focuses on the rational design of bi-functional metal promoted zeolites catalysts for the conversion of NGLs to value added chemicals, such as, olefins, aromatics, and nitriles through dehydrogenation, aromatization, and ammoxidation. We are interested in unraveling the kinetics, mechanistic steps, and structure/performance relationships for the new or existing catalytic systems through the Transient Kinetic Analysis or Chemical Relaxation.

Transient Kinetic Analysis
The chemical relaxation type Transient Kinetic experiments, such as the Steady State Isotopic Transient Kinetic Analysis (SSITKA) and Chemical Transient Kinetic (CTK), are one of the most powerful techniques to obtain valuable mechanistic and kinetic information, such as the abundancy of catalyst-bound reaction intermediates at a molecular level and their reactivity “k” (rate constant). Such experiment provides quantitative information about construction of the catalytically active phase until the steady state is reached.
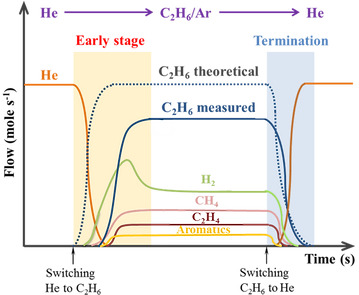
An example of the scheme for the Transient Kinetics study of ethane dehydroaromatization.
Fundamental of Transient Kinetic Analysis:
In a typical reaction A to B with only one type of intermediate [I*]:
At steady state, the mass balance of I* gives as the following:
However, during the Transient Kinetic experiment, we are able to remove the reactant A abruptly from the reactor system (by replacing A with inert gas). Then the rate of production of I*=0. Therefore, the mass balance of I* can be simplified to:
When assuming a pseudo first order reaction kinetics and the discontinuing the addition of A to the feed stream does not affect the value of k2:
With removal of A taking place at t=0, this equation integrates into:
and therefore:
As a result, the concentration of intermediate I* decrease exponentially, which can be observed from the decay in the product B during the transient experiment. Then the characteristic time constant of the decay of product B equals to 1/k2. Therefore, the value of k2 can be determined.